Abstract
Background: The identification of mutations in IDH1 and IDH2 in ~20% of AML pts has ushered in the modern era of precision medicine in AML. The functional implications of the resulting neomorphic activity of these mutated enzymes, has resulted in FDA-approved targeted therapies. Similarly, the changes in methylation due to IDH mutations (IDHm) have shown high responses with HMA-based regimens. In the frontline setting, where traditional IC regimens are also used, no clear guidance exists which choice elicits the best outcomes for IDHm pts. Furthermore, emerging data on the importance of the biologic context on the response to different agents, including co-existing gene mutations and pt age, pose additional questions that need to be systematically addressed in order to determine the best-individualized approach. We set out to address this question, and provide data-driven treatment decision support for the ~20% of AML pts harboring IDHm.
Methods: Using the AML pt collection from the Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology (Alliance, 1986-2013), and a new multicenter collaboration between four major US Cancer Centers (consecutive pts, 2015-2019), we have assembled the thus far largest cohort of 804 IDH1/2m adult AML pts, treated with standard 7+3 IC (n=578), IDH-directed inhibitors (IDHi, n=58) or HMA (without IDH2i or BCL2i, n=75). We investigated the role of different IDH1/2m, co-mutational patterns, and clinical features in predicting response to different frontline therapies.
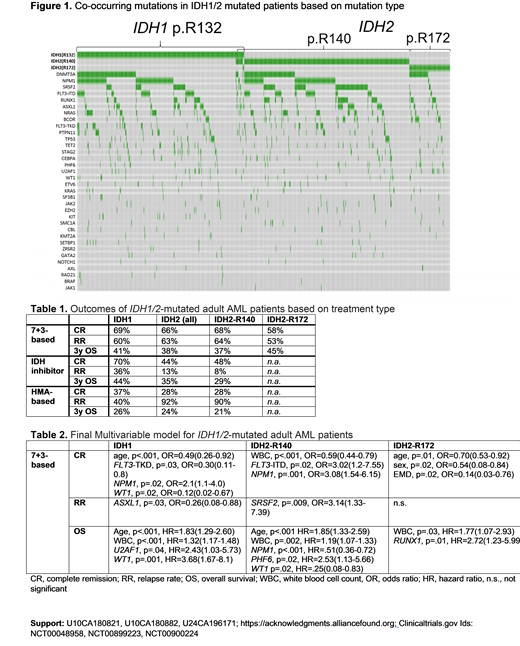
Results: IDH1/2m pts were predominantly older, with 64% aged ≥60 y. Nineteen percent of pts presented with extramedullary disease (EMD) at diagnosis. Pts with all IDHm mutation types commonly harbored DNMT3Am (IDH1-R132m, 38%; IDH2-R140m, 31%; IDH2-R172m, 48%), but differed with respect to other co-occurring mutations (Fig. 1). IDH1m pts most frequently had mutations in the NPM1 (43%), FLT3-ITD (19%), SRSF2 (15%), and NRAS (14%) genes. IDH2-R140m pts harbored mutations in NPM1 (37%), SRSF2 (33%), FLT3-ITD (19%) and RUNX1 (16%) most often, whereas IDH2-R172m pts frequently had BCORm (21%) and RUNX1m (20%), but rarely harbored FLT3-ITD (6%) or NPM1m (2%).
Clinical outcomes had notable differences in complete remission (CR) rates, relapse rates (RR) and overall survival (OS), both with respect to IDHm-type, and also frontline therapy (Table 1). IDH1m pts treated with IC (n=239) had a CR rate of 69%. The CR rates were differentially impacted by clinical characteristics and co-occurring mutations, which were identified in multivariate analysis (MVA; positive prognosticator [PP] for CR: NPM1m; negative prognosticator [NP]: WT1m, FLT3-TKD, higher age, Table 2). IC-treated IDH1m pts had a RR of 60% (NP: ASXL1m), with a 3y-OS of 41% (NP: U2AF1m, WT1m, higher age, higher WBC). When treated with IDH1i (n=20), pts had a high CR rate of 70%, RR of 36%, and a 3y-OS of 44%. In contrast, pts treated with HMAs had a low CR rate of 37% (NP: higher BM blast %), and 3y-OS of 26%.
IDH2-R140m pts treated with IC (n=231) had a CR rate of 68% (PP: NPM1m, FLT3-ITD NP: higher WBC), RR of 64% (NP: SRSF2m), with a 3y-OS of 37% (PP: NPM1m, WT1m; NP: PHF6m, higher age, higher WBC). The positive prognostic association of FLT3-ITD for CR achievement was surprising, but seemed to be independent of co-occurring NPM1m, with CR rates for pts with NPM1wt/ITD+: 70%, NPM1wt/ITD-: 57%, NPM1m/ITD+:83%, and NPM1m/ITD-:76%.
When treated with IDH1i (n=27), pts had a CR rate of 48%, RR of 8%, with a 3y-OS of 29%. Again, pts treated with HMAs had a low CR rate of 28%, RR of 90% and 3y-OS of 21%. IDH2-R172m pts treated with IC (n=66) had a relatively low CR rate of 58% (NP: higher age, male sex, presence of EMD), RR of 53%, but a relatively high 3y-OS rate of 45% (median: 2.5y; NP: RUNX1m, higher WBC). The number of IDH2i- or HMA-treated pts with IDH2-R172m was too small for analyses.
Conclusions: Given the relatively high response rates to IC of IDH1/2m pts, consideration of co-occurring mutations or clinical features (eg, WT1m or FLT3-TKD as NP for IDH1m, SRSF2m for IDH2-R140m or EMD for IDH2-R172m pts) may help guide frontline treatment decisions. Likewise, encouragingly high response and survival rates of pts treated with frontline IDHi should also factor into decision-making. As more information on high response and survival rates with HMA-based combination regimens comes forth, we will be adding these pts to our on-going analysis.
*first: UB,PP,CT; #last:ASM,KS,AKE
Borate: Jazz Pharma: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy. Talati: AbbVie: Honoraria; Astellas: Speakers Bureau; Jazz: Speakers Bureau; Pfizer: Honoraria; BMS: Honoraria. Madanat: Onc Live: Honoraria; Blue Print Pharmaceutical: Honoraria; Stem line pharmaceutical: Honoraria; Geron Pharmaceutical: Consultancy. Blachly: KITE: Consultancy, Honoraria; INNATE: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Walker: Karyopharm Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Marcucci: Agios: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings. Blum: Leukemia and Lymphoma Society: Research Funding; Forma Therapeutics: Research Funding; Xencor: Research Funding; Nkarta: Research Funding; Celyad Oncology: Research Funding; AmerisourceBergen: Honoraria; Abbvie: Honoraria; Syndax: Honoraria. Larson: Novartis: Research Funding; Takeda: Research Funding; CVS/Caremark: Consultancy; Gilead: Research Funding; Astellas: Consultancy, Research Funding; Epizyme: Consultancy; Rafael Pharmaceuticals: Research Funding; Cellectis: Research Funding. Stone: GlaxoSmithKline: Consultancy; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Boston Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Aprea: Consultancy; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Foghorn Therapeutics: Consultancy; Gemoab: Membership on an entity's Board of Directors or advisory committees; Onconova: Consultancy; Innate: Consultancy; Janssen: Consultancy; Novartis: Consultancy, Research Funding; Jazz: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy; Macrogenics: Consultancy. Byrd: Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, Syndax: Consultancy, Honoraria; Newave: Membership on an entity's Board of Directors or advisory committees; Vincerx Pharmaceuticals: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Mims: Xencor: Research Funding; Leukemia and Lymphoma Society's Beat AML clinical study: Consultancy, Research Funding; Aptevo: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Glycomemetics: Research Funding; Kartos Pharmaceuticals: Research Funding; Genentech: Consultancy; Abbvie: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Syndax Pharmaceuticals: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo: Research Funding. Sweet: Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Eisfeld: Karyopharm (spouse): Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal